Synthesis of Ag@SiO2 hybrid nanoparticles templated by a Triton X-100)/1-hexanol/cyclohexane/H2O water-in-oil microemulsion†
Abstract
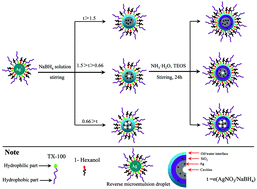
A water-in-oil microemulsion was prepared by using nonionic surfactant Triton X-100, 1-hexanol, cyclohexane and AgNO3 aqueous solution with a concentration of 0.2 mol L−1. Successive addition of sodium borohydride (NaBH4), ammonium hydroxide (NH3·H2O) and tetraethyl orthosilicate (TEOS) to this microemulsion leads to the formation of hierarchically-organized Ag@SiO2 hybrid nanoparticles with Ag nanocrystals randomly distributed inside amorphous SiO2, as proved by transmission electron microscopy (TEM) and high-resolution electron microscopy (HRTEM) observations. In many cases, H2 gas bubbles, which were produced during the reduction of Ag+, were found to influence the structures of Ag nanocrystals. The morphologies of the hybrid nanoparticles and the H2-induced cavities can be easily tuned by the molar ratio of AgNO3 to NaBH4 and the volume ratio of AgNO3 aqueous solution to TEOS. Embedded in amorphous SiO2, the Ag nanoparticles are highly stable, while the unprotected Ag nanocrystals underwent fast aggregation. The inner Ag nanocrystals have dominant (111) planes and are optically active, as shown by X-ray powder diffraction (XRD) and UV-vis measurement, respectively. These properties make these Ag@SiO2 hybrid nanoparticles fascinating candidates for a variety of applications in catalysis and life science.

 Please wait while we load your content...
Please wait while we load your content...