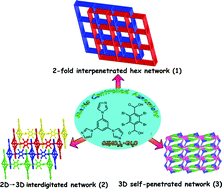
By adjusting the molar ratio of the reactant metal salt and ligands, three new entangled coordination polymers, [Cd(tib)(tbta)(H2O)]n (1) and [Cd(Htib)(tbta)·Htbta·2CH3OH]n (2) and [Cd2(tib)2(tbta)2(H2O)·10H2O]n (3) (tib = 1,3,5-tris(1-imidazolyl)benzene and H2tbta = tetrabromoterephthalic acid), have been prepared and structurally characterized by X-ray diffraction analyses. Complex 1 is a 2-fold interpenetrated three-dimensional (3D) 8-connected uninodal net with rare hex topology. Complex 2 presents a 2D 63-hcb network, which is interdigitated with each other to form the 3D supramolecular framework stabilized hydrogen bonds and halogen bonds. Interestingly, an unprecedented hydrogen-bonded [(Htbta)2(CH3OH)4] cluster was unmasked in the void of 2. Complex 3 is a complicated 3D self-penetrated framework with a point symbol of {4·6·8}{4·65·84}{66}, which can be seen as a pair of 2-fold interpenetrated networks by breaking bidentate-bridging tib ligand. The comparative study revealed that 1–3 are particularly sensitive to the metal–ligand ratio in this system. The thermal stabilities and photoluminescence behaviors of 1 and 2 were also discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...