Orthohalogen substituents dramatically enhance hydrogen bonding of aromatic ureas in solution†
Abstract
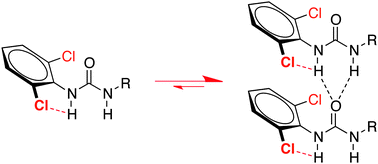
The phenylurea moiety is a ubiquitous synthon in supramolecular chemistry. Here we report that the introduction of chlorine or bromine atoms in the ortho positions to the urea unit is a simple and very efficient way to improve its intermolecular hydrogen bond (HB) donor character. This effect was demonstrated in solution both in the context of self-association of bis-ureas and hydrogen bonding of mono-ureas to strong HB acceptors.

 Please wait while we load your content...
Please wait while we load your content...