Intramolecular arylation of amino acid enolates†
Abstract
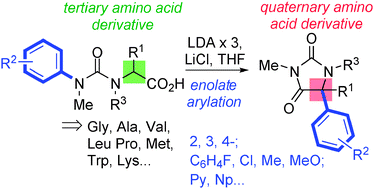
Dianionic enolates formed from N′-aryl urea derivatives of amino acids undergo intramolecular C-arylation by attack of the enolate anion on the N′-aryl ring, leading to a hydantoin derivative of a quaternary amino acid. In situ IR studies allow identification of four intermediates on the reaction pathway.

 Please wait while we load your content...
Please wait while we load your content...