Enantioselective synthesis of α-nitro-δ-ketosulfones via a quinine–squaramide catalyzed conjugate addition of α-nitrosulfones to enones†
Abstract
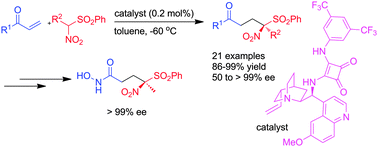
Conjugate addition of α-nitrosulfones to vinyl ketones in the presence of 0.2 mol% of a quinine–squaramide organocatalyst afforded α-nitro-δ-ketosulfones possessing a tetrasubstituted chiral center in excellent yield and enantioselectivity in most cases. This strategy also offers a facile and convenient entry into γ-sulfonylhydroxamates that are one carbon homologs of potent enzyme inhibitors.

 Please wait while we load your content...
Please wait while we load your content...