Anti-Markovnikov hydrophosphoroselenoylation of alkenes using phosphorodiselenoic acid esters leading to the formation of phosphonoselenoic acid esters†
Abstract
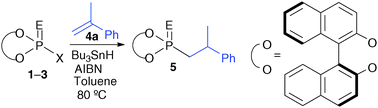
Phosphorodiselenoic acid esters with a binaphthyl group were reacted with alkenes in the presence of Bu3SnH and AIBN to give phosphonoselenoic acid esters in moderate to good yields. The addition of a phosphoroselenoyl group to alkenes proceeded in an anti-Markovnikov fashion. The diastereoselectivity was improved by the introduction of substituents to 3,3′-positions of a binaphthyl group.
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST

 Please wait while we load your content...
Please wait while we load your content...