Synthetically useful noncatalytic strategy: a stereocontrolled rapid cyclization of a three component system to afford hexahydropyrrolizines†
Abstract
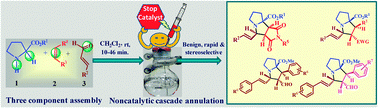
We demonstrate a highly regio- and stereoselective annulation strategy involving unprecedented C2–H and N–H of proline esters, α,β-unsaturated aromatic aldehydes and deactivated olefins without a catalytic aid to afford medicinally important pyrrolo[3,4-a]pyrrolizines and hexahydropyrrolizines under benign reaction conditions.

 Please wait while we load your content...
Please wait while we load your content...