Finding the right path: Baldwin “Rules for Ring Closure” and stereoelectronic control of cyclizations
Abstract
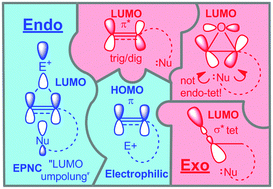
Truly important scientific breakthroughs often come from catching the glimpses of order in chaos and distilling a large body of disjointed data into a clear set of simple concepts. Even more impressive is when the new conceptual framework is created with some of the keystones still missing. Such bold predictions, i.e. Mendeleev's eka-silicon, challenge and inspire, serving as powerful catalysts for scientific growth. The rules for ring closure formulated by Sir Jack Baldwin in 1976 constitute one of such bold intellectual advances. Baldwin developed a classification system that brought order to the chaos of possible cyclization patterns and suggested a set of rules to define the favourable modes of ring closure. Where sufficient data was lacking, particularly for the cyclizations of alkynes, Baldwin made testable predictions that challenged theoretical and experimental chemists. These guidelines have become the common starting point in the design of new cyclization reactions and catalyzed the development of modern stereoelectronic concepts.
- This article is part of the themed collection: Viewpoints

 Please wait while we load your content...
Please wait while we load your content...