Lewis acid-catalyzed regioselective synthesis of chiral α-fluoroalkyl aminesvia asymmetric addition of silyl dienolates to fluorinated sulfinylimines†
Abstract
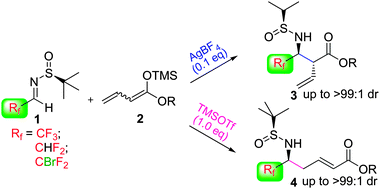
A tunable and highly regio- and diastereoselective addition of acyclic silyl dienolates 2 to several α-fluoroalkyl sulfinylimines 1 was developed. By appropriate choice of the Lewis acid

 Please wait while we load your content...
Please wait while we load your content...