Enantioselective 1,3-dipolar cycloaddition of methyleneindolinones and N,N′-cyclic azomethine imines†
Abstract
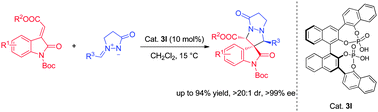
A new chiral bis-phosphoric acid 3l bearing triple axial chirality was synthesized and applied to effect a highly enantioselective 1,3-dipolar cycloaddition reaction between N,N′-azomethine

 Please wait while we load your content...
Please wait while we load your content...