Resonance-assisted hydrogen bonding induced nucleophilic addition to hamper ESIPT: ratiometric detection of cyanide in aqueous media†‡
Abstract
For ratiometric “naked eye” detection of CN−, an ESIPT exhibiting
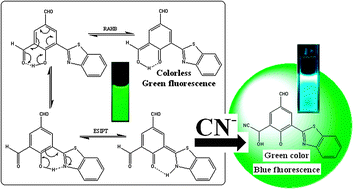
- This article is part of the themed collections: ChemComm 60th Anniversary Historic Papers from India and In Celebration of Andrew D. Hamilton’s Career in Chemistry

 Please wait while we load your content...
Please wait while we load your content...