Cu(ii)-catalyzed cyclization of α-diazo-β-oxoamides with amines leading to pyrrol-3(2H)-ones†
Abstract
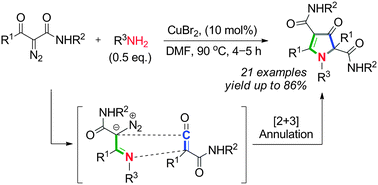
A novel Cu(II)-catalyzed cyclization of α-diazo-β-oxoamides with amines has been developed, constituting a straightforward method to construct pyrrol-3(2H)-one rings. The intramolecular hydrogen bonding effect in α-diazo-β-oxoamides plays an essential role in this reaction. A plausible reaction mechanism involving divergent generation and subsequent [2 + 3] cyclization of ketene and α-diazoimine intermediates was proposed.

 Please wait while we load your content...
Please wait while we load your content...