Redox active aluminium(iii) complexes convert CO2 into MgCO3 or CaCO3 in a synthetic cycle using Mg or Ca metal†‡
Abstract
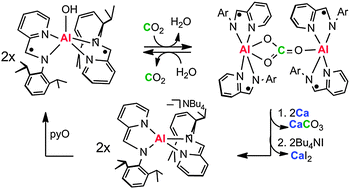
Redox-active Group 13 molecules possess the unusual combination of concomitant redox and acid–base reactivity. These combined properties enable regeneration of a metal
- This article is part of the themed collections: Celebrating the ChemComm Emerging Investigator Lectureship Winners and Emerging Investigators 2013

 Please wait while we load your content...
Please wait while we load your content...