Oxidation of water by a nonhaem diiron(iv) complex via proton-coupled electron transfer
Abstract
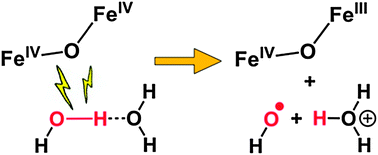
A high-potential nonhaem (μ-oxo)diiron(IV) complex was found to oxidize water to a hydroxyl radical via PCET, instead of forming an O–O bond. The rate-determining step requires a second water molecule, proposed to act as a base to promote proton transfer. This work shows that additional factors besides a high redox potential are required to effect O–O bond formation.
- This article is part of the themed collection: Biological oxidation reactions: mechanisms and design of new catalysts

 Please wait while we load your content...
Please wait while we load your content...