Hydrophilic interaction/cation-exchange chromatography for glycopeptide enrichment by using a modified strong-cation exchange material†
Abstract
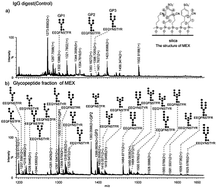
Protein glycosylation analysis based on mass spectrometry (MS) remains a major analytical challenge, because of the low proportions of glycopeptides in the glycoprotein digest and the suppression effect of the coexisting non-glycosylated peptides. Therefore, selective enrichment of glycopeptides prior to MS is of great significance. In this study, we presented hydrophilic interaction/cation-exchange chromatography (HILIC/CEX) for glycopeptide enrichment by applying a customized material named MEX. MEX is a silica-based material bonded with two types of functional groups, benzenesulfonic acid and cyano-propyl groups. Compared with traditional strong-cation exchange (SCX) columns, moderate retention of analytes can be obtained on MEX due to the decreased ion-exchange capacity caused by the introduction of the cyano-propyl groups. Meanwhile, superior HILIC selectivity was also exhibited on MEX. In glycosylation analysis, MEX exhibited high selectivity and broad coverage for glycopeptides, which indicated the promising potential of the MEX material in glycosylation analysis.

 Please wait while we load your content...
Please wait while we load your content...