Development of supercritical fluid (carbon dioxide) based ultra performance convergence chromatographic stability indicating assay method for the determination of clofarabine in injection
Abstract
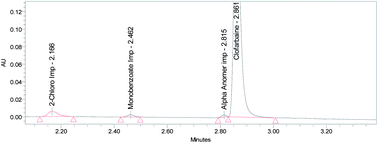
The present study reports the development and validation of a stability indicating assay method for clofarabine in injection on a UPC2™ (ultra performance convergence chromatography) instrument, which utilizes the unrealized potential of supercritical fluid chromatography. The use of UPC2™ provides a single viable technique that is a sustainable, reduced cost, and green technology that lowers the use of organic solvents. Based on this advantage, we explored a simple and robust method in order to increase sample throughput and productivity to quantify clofarabine in the presence of its potential impurities and other degradants. The separation was achieved on a BEH-2-ethyl pyridine (BEH 2EP) column (100 mm × 3.0 mm I.D. with an average pore diameter of 1.7 μm) by using methanol as a co-solvent and carbon dioxide as a mobile phase in the ratio of 30 : 70. The detection is carried out at a wavelength of 254 nm. We are able to achieve the separation of clofarabine from its potential impurities and other degradants in less than 6 minutes with a low amount of solvent consumption. The new method is validated in accordance with the ICH-guidelines and exhibited good intra- and inter-day precision, accuracy and linearity (r2 ≥ 0.999) over a range of 50% to 150% of target concentration.

 Please wait while we load your content...
Please wait while we load your content...