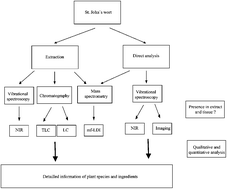
In the present study, a novel analytical platform is introduced, which enables both analysis and quality control of St John's wort extracts and tissue. The synergistic combination of separation techniques (including thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC)) with mass spectrometry (MS) and vibrational spectroscopy is demonstrated to get deeper insights into the ingredients composition. TLC was successfully employed to identify some unknown ingredients being present in samples with Chinese provenience. On the one hand, the novel HPLC method described here allowed us to clearly differentiate between European and Chinese samples; on the other hand, this method could successfully be employed for the semi-preparative isolation of an unknown ingredient. Matrix-free laser desorption ionization time of flight mass spectrometry (mf-LDI-TOF/MS) using a specially designed titanium oxide layer was employed to identify the structure of the substance. The analytical knowledge generated so far was used to establish an infrared spectroscopic model allowing both quantitative analysis of ingredients as well as differentiating between European and Chinese provenience. Finally, infrared imaging spectroscopy was conducted to obtain knowledge about the highly resolved distribution of ingredients. The analytical platform established so far can be used for fast and non-destructive quantitation and quality control to identify adulteration being of interest according to the Deutsche Arzneimittel Codex (DAC).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...