Differential proteomic analysis of caveolin-1 KO cells reveals Sh2b3 and Clec12b as novel interaction partners of caveolin-1 and Capns1 as a potential mediator of caveolin-1-induced apoptosis†
Abstract
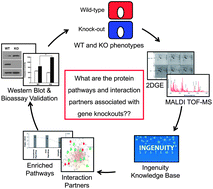
Caveolin-1 (Cav1) is a small scaffolding protein involved in a variety of cellular functions, including cell signaling, lipid transport and membrane traffic. The objective of this study was to use comparative proteomics to identify differentially expressed proteins in Cav1 knockout (KO) mouse embryonic fibroblasts. These deregulated proteins were then analyzed using systems biology tools to gain insight into the local network properties and to identify the interaction partners of Cav1. We identified five proteins that were up-regulated and ten proteins that were down-regulated in Cav1 KO cells, suggesting that the local network behaves as a complex system. Protein interaction network analysis revealed two proteins, Sh2b3 and Clec12b, as novel interaction partners of Cav1. Functional annotation showed apoptosis signaling as the most significant pathway. To validate this functional annotation, Cav1 KO cells showed more than 1.5-fold increase in caspase-3 activity over wild type cells upon apoptotic stimulation. We also found that calpain small subunit 1 is up-regulated in Cav1 KO cells and directly influences the cell response to apoptotic stimuli. Moreover, Capns1 was reduced in Cav1 KO cells following re-expression of Cav1, and suppression of Capns1 expression in Cav1 KO cells significantly inhibited the cells to apoptotic stimuli, as measured by caspase 3 activity. In conclusion, our results suggest that Sh2b3 and Clec12b functionally interact with Cav1 and that calpain small subunit 1 may mediate Cav1-induced apoptosis.

 Please wait while we load your content...
Please wait while we load your content...