Tyrosine-functionalized CuInS2 quantum dots as a fluorescence probe for the determination of biothiols, histidine and threonine†
Abstract
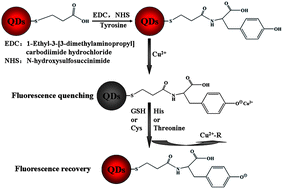
A novel, rapid and highly sensitive fluorescence turn-on assay for the detection of biothiols (glutathione, and L-cysteine), histidine and threonine was developed. Water-soluble CuInS2 ternary quantum dots (QDs) capped by mercaptopropionic acid (MPA) were directly synthesized in aqueous solution, and then functionalized using tyrosine molecules to form tyrosine-functionalized CuInS2 QDs (T-CuInS2 QDs). The fluorescence of T-CuInS2 QDs would decrease in the presence of Cu2+ due to the coordination effect of phenolic hydroxyls of the tyrosine molecules. Subsequently, the addition of biothiols (glutathione and L-cysteine), histidine or threonine could turn on the fluorescence of the T-CuInS2 QDs–Cu2+ system due to their strong affinity for Cu2+. The proposed method was simple in design and fast in operation, and it was applied for the detection of glutathione, L-cysteine, histidine, and threonine in human serum samples with satisfactory results.

 Please wait while we load your content...
Please wait while we load your content...