Radical induced disulfide bond cleavage within peptides via ultraviolet irradiation of an electrospray plume†
Abstract
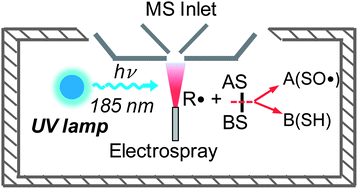
Radical induced disulfide bond cleavage in peptides was demonstrated by ultraviolet (UV) radiation of the electrospray ionization (ESI) plume using a low pressure mercury (LP-Hg) lamp. Tandem mass spectrometry and accurate mass measurements confirmed that the primary reaction products were due to disulfide bond cleavage to form thiol (–SH) and sulfinyl radical (–SO˙). Mechanistic studies showed that the 185 nm emission from a LP-Hg lamp was responsible for UV photolysis of atmospheric O2, which further initiated secondary radical formation and subsequent disulfide bond cleavage by radical attack. The radical induced disulfide bond cleavage was found to be analytically useful in providing rich sequence information for naturally occurring peptides containing intrachain disulfide bonds. The utility of this method was also demonstrated for facile disulfide peptide identification and characterization from protein digests.

 Please wait while we load your content...
Please wait while we load your content...