N-phosphorylation labeling for analysis of twenty natural amino acids and small peptides by using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry†
Abstract
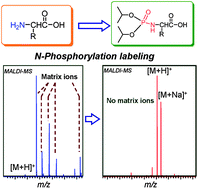
N-phosphorylation labeling was utilized to analyze the low molecular weight compounds by using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). A wide range of natural amino acids and short peptides was successfully analyzed by MALDI-TOF MS without matrix background interferences. The N-phosphorylation labeling reaction was carried out easily within 30 min in a one-pot reaction under mild reaction conditions. The phosphoryl derivatization reaction is a global labeling approach with high selectivity and high specificity with targeting only on the N-terminal and ε-amino group of Lys. The incorporation of a neutral phosphoryl group with high gas-phase affinity of protons not only improves the ionization efficiency of target molecules and simultaneously decreases the ion suppression effects in MALDI-TOF MS analysis, but also greatly reduces or eliminates the matrix background interferences by suppressing the matrix signals and increasing the molecular weight of the targeted compounds. By applying the N-phosphorylation labeling approach, many amino acids could be detected in serum samples by using MALDI-TOF MS.

 Please wait while we load your content...
Please wait while we load your content...