Synthesis and linear and nonlinear optical properties of low-melting π-extended porphyrins†
Abstract
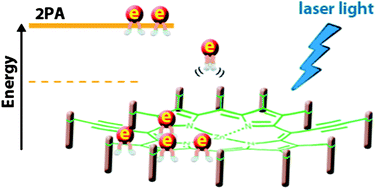
A large and diverse library of trans-A2B2 and A2BC-porphyrins possessing two arylethynyl substituents at the meso positions has been efficiently synthesized and tested for their two-

 Please wait while we load your content...
Please wait while we load your content...