Oligofuran-containing molecules for organic electronics†
Abstract
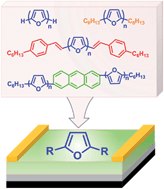
We describe the synthesis, characterization and field effect transistor (FET) properties of a series of furan-based conjugated oligomers such as unsubstituted, hexyl- and styryl-capped linear oligofurans and oligofuran-substituted anthracene derivatives. All studied oligofurans show high fluorescence and good thermal stability. Top contact organic FETs (OFETs) fabricated with oligofurans as the active layer show hole mobilities (∼0.01 to 0.07 cm2 V−1 s−1) and on/off ratios (104 to 106) on a par with the corresponding oligothiophene analogues, while the threshold voltages displayed by oligofuran-based OFETs are significantly reduced due to higher HOMO energies as compared to those of oligothiophenes. Electroluminescence observed in oligofuran-based OFETs in a bottom-contact geometry is limited by electron injection. Overall, we find that furan building blocks are excellent candidates for replacing thiophene in optoelectronic materials.

 Please wait while we load your content...
Please wait while we load your content...