Perfluoroalkyl-substitution versus electron-deficient building blocks in design of oligothiophene semiconductors†
Abstract
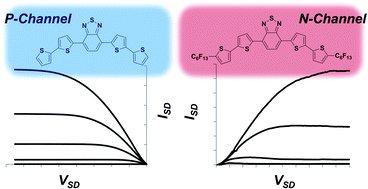
A series of oligothiophenes containing either electron withdrawing perfluorohexyl substituents or an electron deficient benzothiadiazole core, or both, were synthesized and their properties were evaluated by optical spectroscopy, cyclic voltammetry and DFT calculations. The charge transport properties of these compounds were studied in field effect transistors, revealing how the majority charge carrier is affected by the chemical substitution. Incorporation of benzothiadiazole significantly lowers the LUMO energy of the oligomers, but does not affect the HOMO. On the other hand, perfluoroalkyl groups lowered both the HOMO and LUMO, and were essential for achieving electron conductivity in this series of compounds.

 Please wait while we load your content...
Please wait while we load your content...