Fast production of β-Ni(OH)2nanostructures with (001) and (100) plane exposure and their electrochemical properties†
Abstract
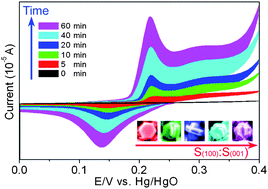
Based on theoretical analysis of the crystal structure of β-Ni(OH)2, the (001) and (100) planes have the highest and the second highest Ni2+

 Please wait while we load your content...
Please wait while we load your content...