Scalable synthesis of hollow Cu2O nanocubes with unique optical properties via a simple hydrolysis-based approach†
Abstract
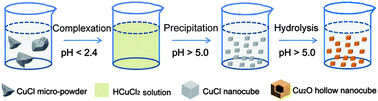
Hydrolysis reactions merely involve a precursor and water, which makes them very attractive for the mass-production of nanomaterials at low cost. In the present study, the behavior of cuprous chloride (CuCl) in water solutions was comprehensively investigated, and the medium pH was found to be critical for engineering the reactions and final products. Accordingly, a facile and efficient process based on pH-controlled hydrolysis was designed to fabricate a unique nanostructure, hollow Cu2O nanocubes. In this process, commercially available CuCl micro-powder is first dissolved in highly acidic water. Then, upon increasing the pH, uniform CuCl nanocubes precipitate and further serve as self-sacrificial templates to produce hollow Cu2O nanocubes via hydrolysis. The synthesis is fast, takes place at room temperature and is solely based on tuning the medium pH. The product exhibits a homogenous size, well-defined shape, surfactant-free surface and excellent optical properties, indicating that hydrolysis-based synthetic routes can be a powerful method for preparing novel nanostructures on a large scale and at low cost.

 Please wait while we load your content...
Please wait while we load your content...