Thermal 8π electrocyclic reaction of heteroarene tetramers: new efficient access to π-extended cyclooctatetraenes†
Abstract
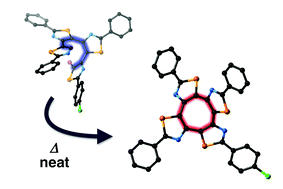
Bromo-substituted acyclic thiazole tetramers selectively underwent an 8π thermal electrocyclic reaction at high temperatures of 280–300 °C without solvent, producing π-extended thiazole-fused cyclooctatetraenes (COTs) in high yields. A quantum chemical study provided a rationale for the lower activation barrier and high selectivity of the 8π electrocyclic reaction mode over a competitive 6π electrocyclic reaction. The terminal bromine atom acts as a leaving group to trigger the rearomatization of the electrocyclized intermediate, which leads to the formation of the energetically stable COT products. This synthetic protocol enables us to synthesize a series of thiazole-fused COTs, including a derivative that can form loosely stacked columnar arrays in the crystalline state and shows fluorescence in the solid state.

 Please wait while we load your content...
Please wait while we load your content...