Monolayers of trimesic and isophthalic acid on Cu and Ag: the influence of coordination strength on adsorption geometry
Abstract
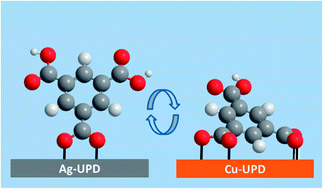
Self-assembled monolayers (SAMs) of isophthalic acid (IPA) and trimesic acid (TMA) were prepared from solution using Au(111)/mica substrates modified by underpotential deposition (UPD) of Cu and Ag. Ex situ analysis by scanning tunneling microscopy (STM), synchrotron based X-ray photoelectron spectroscopy (XPS) and near-edge X-ray absorption fine structure (NEXAFS) spectroscopy reveals pronouncedly different film structures for the (1 × 1) Cu and (1 × 1) Ag UPD-modified Au(111)/mica substrates. On Cu, both IPA and TMA form highly crystalline commensurate layers with a characteristic row structure, a significant tilt of the aromatic ring by ∼50° from the surface normal and a bipodal adsorption geometry of the molecules involving two carboxylate moieties. In contrast, a significantly smaller tilt angle of ∼20° and a monopodal adsorption geometry is found on Ag. Even though a row structure is also observed for TMA on Ag, it lacks the regularity and, thus, commensurability found on Cu. Contrasting the other systems which yield molecularly resolved STM images, no lateral order is seen in the IPA SAMs despite the fact that both the IPA and TMA layers on Ag have analogous structures with essentially the same tilt angle of the benzene ring. The difference between IPA and TMA on Ag is explained by the difference in the number of carboxyl groups available for stabilising the layer via intermolecular hydrogen bonds.
- This article is part of the themed collection: Physical Chemistry

 Please wait while we load your content...
Please wait while we load your content...