Concise total synthesis of (+)-bionectins A and C†
Abstract
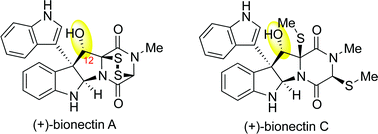
The concise and efficient total synthesis of (+)-bionectins A and C is described. Our approach to these natural products features a new and scalable method for erythro-β-hydroxytryptophan amino acid synthesis, an intramolecular Friedel–Crafts reaction of a

 Please wait while we load your content...
Please wait while we load your content...