Reversible blocking of antibodies using bivalent peptide–DNA conjugates allows protease-activatable targeting†
Abstract
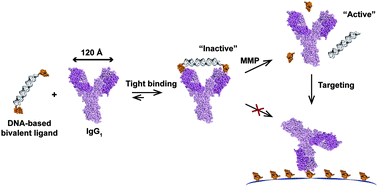
Antibody-based molecular recognition plays a dominant role in the life sciences ranging from applications in diagnostics and molecular imaging to targeted

 Please wait while we load your content...
Please wait while we load your content...