MegaStokes BODIPY-triazoles as environmentally sensitive turn-on fluorescent dyes†
Abstract
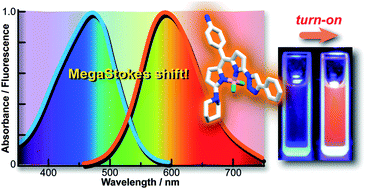
A novel class of triazole-derivatized BODIPY compounds have been synthesized on solid-phase by employing mild reaction conditions based on the copper-catalyzed azide–alkyne cycloaddition. The resulting BODIPY-triazoles exhibited MegaStokes shifts (up to 160 nm) and remarkable environmentally sensitive quantum yield increments that asserted their potential as turn-on fluorescent sensors. Out of a library of 120 compounds, we identified BDC-9 as a fluorescent chemosensor with high sensitivity and remarkable species-selectivity towards human serum albumin. These results validate MegaStokes BODIPY dyes as new fluorophores for the development of environmentally sensitive fluorescent probes.

 Please wait while we load your content...
Please wait while we load your content...