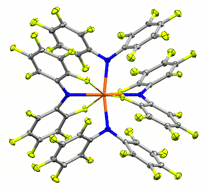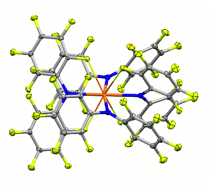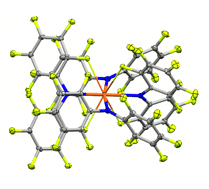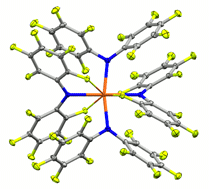
The fluorinated diarylamines HNPhPhF, HNPhF2, HNPhArF, PhF = 2,3,4,5,6-pentafluorophenyl, ArF = 3,5-bis(trifluoromethyl)phenyl, are used to prepare complexes of uranium(III, IV) ions. Despite being electron-poor amines with little steric bulk, their coordinated amide ligands exhibit direct control over the coordination environment through a subtle, cooperative interplay of multiple labile F→U dative interactions and favorable arene–arene interactions. The C–F→U interactions, ∼8.9 kcal mol−1 as determined by variable temperature NMR experiments, persist in solution and allow the isolation of otherwise unstable species as well as the first pseudo-square planar uranium complex.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...