Influence of adsorbed gas at liquid/solid interfaces on heterogeneous cavitation†
Abstract
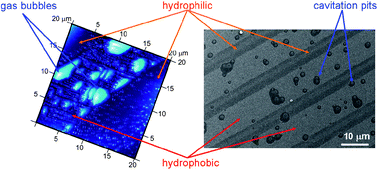
The presence of dissolved gas at the solid/liquid interface can play a crucial role in heterogeneous cavitation. Here we focus our attention on the relationship between gas conditions and cavitation nucleation at planar solid surfaces with alternating hydrophobic/hydrophilic properties.

 Please wait while we load your content...
Please wait while we load your content...