Dynamic combinatorial chemistry in a solvothermal process between nickel(ii), halides and organosulphur ligands†
Abstract
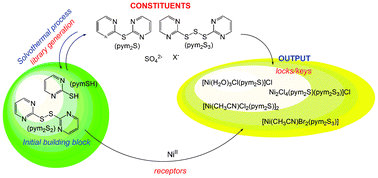
A dynamic combinatorial process has allowed the isolation of four new compounds produced in the direct solvothermal reactions of

 Please wait while we load your content...
Please wait while we load your content...