Conformational analysis of 6-fluorosalicylic acid†
Abstract
Salicylic acid is the precursor of an important analgesic drug, and fluorination may modify its properties, including pKa in solution, viscosity, polarity and molecular conformation. The intramolecular hydrogen bond is an interaction that modifies the conformational equilibrium of molecules and, therefore, it can modify their macroscopic properties, such as bioactivity. Competition between F and N as proton acceptors for the acidic COOH hydrogen in 6-fluoroanthranilic acid could not be experimentally probed earlier because of, among other factors, its tendency to form a zwitterionic species due to the higher basicity of the nitrogen. In 6-fluorosalicylic acid (1), the fluorine atom competes with oxygen for the COOH proton, thus affecting the conformational equilibrium when compared to salicylic acid itself. Indeed, 1 shows two major conformers in the gas phase and solution, while the isomer 4-fluorosalicylic acid is expected to exhibit only one among three possible conformers. Theoretical calculations indicate that the second most stable conformer of 1 exhibits intramolecular F⋯H(OOC) hydrogen bonds, which is corroborated by the small, but detectable correlation between 19F and 1H(OOC) in the HETCOR NMR spectrum in benzene-d6 solution.
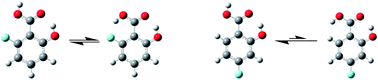

 Please wait while we load your content...
Please wait while we load your content...