Amidoxime porous polymers for CO2 capture
Abstract
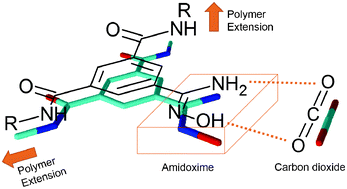
CO2 capture from fossil fuel based electricity generation remains costly since new power plants with monoethanol amine (MEA) as the scrubbing agent are under construction. Amidoximes are known to mimic MEA, and porous polymers with amidoximes could offer a sustainable solution to carbon capture. Here we report the first amidoxime porous polymers (APPs) where aromatic polyamides (aramids) having amidoxime pendant groups were synthesized through low temperature condensation of 4,4′-oxydianiline (ODA) and p-phenylene diamine (p-PDA) with a new type of nitrile-bearing aromatic diacid chloride. The nitrile pendant groups of the polyamides were converted to an amidoxime functionality by a rapid hydroxylamine addition (APP-1 and APP-2). The CO2 adsorption capacities of these polyamides were measured at low pressure (1 bar) and two different temperatures (273 and 298 K) and high pressure (up to 225 bar – the highest measuring pressure to date) at 318 K. The low pressure CO2 uptake of APP-1 was found to be 0.32 mmol g−1 compared with APP-2 (0.07 mmol g−1) at 273 K, whereas at high pressure they showed a substantial increase in CO2 adsorption capacity exhibiting 24.69 and 11.67 mmol g−1 for APP-1 and APP-2 respectively. Both aramids were found to be solution processable, enabling membrane applications.

 Please wait while we load your content...
Please wait while we load your content...