Facile and efficient N-arylation of amino acid esters with (−)-methyl-3-dehydroshikimiate(3-MDHS): a bio-based and metal-free strategy leading to N-aryl amino acid derivatives†
Abstract
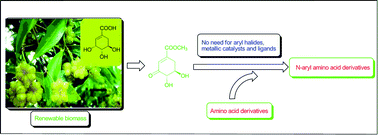
A novel and convenient bio-based protocol for the preparation of N-aryl amino acid derivatives from (−)-methyl-3-dehydroshikimate and

 Please wait while we load your content...
Please wait while we load your content...