Encapsulation of lipase in mesoporous silica yolk–shell spheres with enhanced enzyme stability†
Abstract
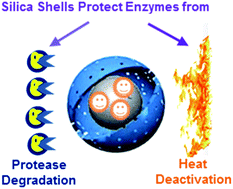
Enzyme encapsulation is an attractive method among the different immobilization strategies to improve the reusability and stability of enzymes because it can separate enzymes from a hazardous external environment. However, current encapsulation methods have limitations including enzyme leakage. In this study, a new approach based on a two-step soft templating method has been proposed to encapsulate lipase within substrate permeable mesoporous silica yolk–shell spheres. In the first step, lipase was immobilized onto epoxy functionalized silica nanospheres that serve as the core materials. The core materials were mixed with a fluorocarbon surfactant, FC4, to form a core–vesicle complex. In the second step, a mesoporous silica shell was assembled surrounding the core–vesicle complex to form the yolk–shell structure with the lipase encapsulated. The mesoporous silica shell has a pore size of 2.1 nm, which is permeable to the reactant and product while isolating the enzymes from harmful external conditions. The encapsulated lipase retained 87.5% of its activity after thermal treatment at 70 °C for 2 hours while the free enzyme lost 99.5% of its activity under the same treatment. Importantly, the encapsulated lipase shows significantly enhanced resistance to degradation by proteases.

 Please wait while we load your content...
Please wait while we load your content...