Toward understanding the growth mechanism of Aun(SR)m nanoclusters: effect of solvent on cluster size
Abstract
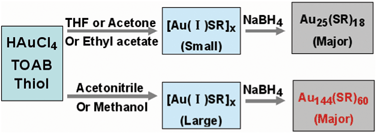
Despite the recent advances in size-controlled synthesis of thiolate-protected Aun(SR)m nanoclusters, understanding the growth mechanism of nanoclusters still significantly lags behind. In this work, we report an important finding that the reaction medium plays a major role in influencing the final cluster size. Specifically, we focus on the one-phase synthetic process that leads to predominant formation of Au25(SR)18 or Au144(SR)60—two ubiquitous nanoclusters in nanocluster synthesis. When THF,

 Please wait while we load your content...
Please wait while we load your content...