A general and green procedure for the synthesis of organochalcogenides by CuFe2O4nanoparticle catalysed coupling of organoboronic acids and dichalcogenides in PEG-400†
Abstract
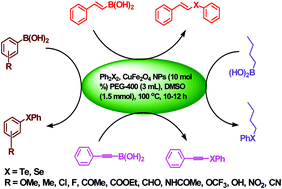
A general and efficient procedure has been developed for the synthesis of organochalcogenides (

 Please wait while we load your content...
Please wait while we load your content...