Azolium hydrogen carbonates and azolium carboxylates as organic pre-catalysts for N-heterocyclic carbene-catalysed group transfer and ring-opening polymerisations†
Abstract
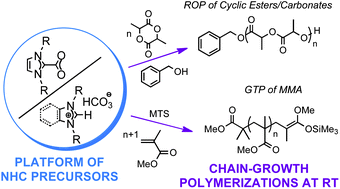
Various imidazolium-2-carboxylates and (benz)imidazolium hydrogen carbonates, denoted as NHC–CO2 adducts and [NHC(H)][HCO3], respectively, were employed as masked N-heterocyclic carbenes (NHCs) to bring about both the ring opening polymerisation (ROP) of D,L-lactide (LA) and the group transfer polymerisation (GTP) of methyl methacrylate (MMA). Polymerisation reactions could be performed at room temperature by generation of related free NHCs by a simple solvation effect. Catalytic efficiencies of imidazolium-2-carboxylates were found to be approximately three times higher than that of their hydrogen carbonate counterparts for the ROP of D,L-LA, except in the particular case of 1,3-dicyclohexylbenzimidazolium hydrogen carbonate that exhibited a similar catalytic performance to that of NHC–CO2 adducts. The catalytic efficiencies of free NHCs and NHC precursors were thus in the following order: [NHC(H)][HCO3] < NHC–CO2 adducts ≪ free NHCs. Only NHC–CO2 adducts allowed the catalysis of the GTP of MMA in bulk in a controlled manner, [NHC(H)][HCO3] precursor salts being poorly soluble in the monomer substrate, causing a loss of control of GTP under solvent-less conditions.

 Please wait while we load your content...
Please wait while we load your content...