Synthesis and self-assembly of amphiphilic polymers based on polyoxazoline and vegetable oil derivatives†
Abstract
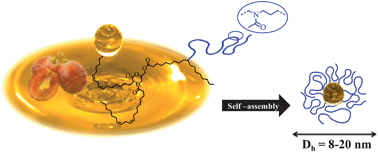
The synthesis and self-assembly of amphiphilic polymers based on unsaturated vegetable oils and poly(2-methyl-2-oxazoline) (POx) are reported. Two architectures of lipopolymers were explored starting from fatty methyl esters and raw vegetable oils. The latter were converted into macroinitiators for cationic ring-opening polymerization (CROP) of 2-methyl-2-oxazoline. Firstly, a thiol–ene coupling reaction in the presence of mercaptoethanol under UV irradiation was performed to introduce hydroxyl groups that were further transformed into initiating species used for cationic polymerization. According to this strategy, various lipopoly(2-methyl-2-oxazoline)s (LipoPOxs) with different lipidic/POx ratios were successfully obtained and characterized by 1H NMR, SEC and MALDI-TOF analyses. Finally, the self-organization of the LipoPOx was studied using dynamic light scattering (DLS). Well-shaped nanoparticles were obtained with characteristic radii of 4.3 and 10.2 nm for the fatty ester- and triglyceride-based lipopolymers, respectively. The latter value is about twice the former one due to the higher lipidic fraction of the triglyceride-based polymer that promotes hydrophobic interactions. The relaxation time distributions of both systems were found to be monomodal, indicating monodisperse colloidal suspensions.

 Please wait while we load your content...
Please wait while we load your content...