Chair interconversion and reactivity of mannuronic acid esters†
Abstract
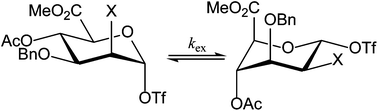
Mannopyranosyluronic acids display a very unusual conformation behavior in that they often prefer to adopt a 1C4 chair conformation. They are endowed with a strikingly high reactivity when used in a glycosylation reaction as a glycosyl donor. To investigate the unusual conformational behavior a series of mannuronic acid ester derivatives, comprising anomeric triflate species and O-methyl glycosides, was examined by dynamic NMR experiments, through lineshape analysis of 1H and 19F NMR spectra at various temperatures from −80 °C to 0 °C. Exchange rates between 4C1 and 1C4 chair conformations were found to depend on the electronic properties and the size of the C2 substituent (F, N3 or OBn) and the aglycon, with higher exchange rates for the glycosyl triflates and smaller C2 substituents. Low temperature 19F exchange spectroscopy experiments showed that the covalently bound anomeric triflates did not exchange with free triflate species present in the reaction mixture. To relate the conformational behavior of the intermediate triflates to their reactivity in a glycosylation reaction, their relative reactivity was determined via competition reactions monitored by 1H NMR spectroscopy at low temperature. The 2-O-benzyl ether compound was found to be most reactive whereas the 2-fluoro compound – the most flexible of the studied compounds – was least reactive. Whereas the ring-flip of the mannuronic acids is important for the enhanced reactivity of the donors, the rate of the ring-flip has little influence on the relative reactivity.

 Please wait while we load your content...
Please wait while we load your content...