The first asymmetric total synthesis of (+)-coriandrone A and B†
Abstract
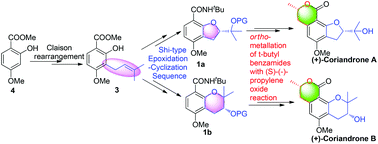
The first enantioselective total synthesis of (+)-coriandrone A and B, two bioactive natural products, has been achieved in 10 steps and 11 steps starting from commercially available methyl 2-hydroxy-4-methoxybenzoate. Key reactions include a Claison rearrangement, a Shi-type epoxidation–cyclization sequence and ortho-metallation of t-butylbenzamides with (S)-(−)-propylene oxide reaction.

 Please wait while we load your content...
Please wait while we load your content...