The anomeric effect on the basis of natural bond orbital analysis
Abstract
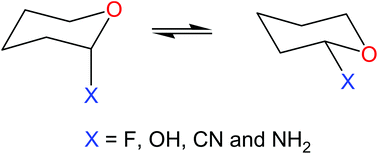
The anomeric effect has been assigned as being due to hyperconjugation, electrostatic/steric interactions or exchange effects; thus, there is no general consensus about its actual origin. The classical hyperconjugation model, usually investigated using natural bond orbital (NBO) analysis, has been arbitrarily refuted because it would not explain some cases of the preferred equatorial anomer over the axial one in polar solution. In this study, hyperconjugation was shown to be dependent on the medium and NBO analysis explains quite well the estimated amounts of axial and equatorial 2-substituted tetrahydropyrans (substituents = F, OH, NH2 and CN) both in the gas phase and aqueous solution. Overall, there is no reason to abandon the hyperconjugation model as it plays a dominating role of the anomeric effect in some systems, while NBO analysis reproduces the energetic profiles on the basis of both hyperconjugative and Lewis-type contributions.

 Please wait while we load your content...
Please wait while we load your content...