Total synthesis and biological evaluation of (−)-exiguolide analogues: importance of the macrocyclic backbone†
Abstract
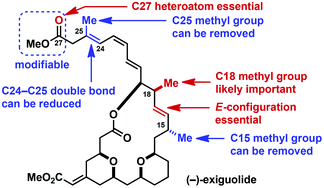
(−)-Exiguolide (1), isolated from the marine sponge Geodia exigua, has been shown to inhibit the growth of the A549 human lung adenocarcinoma and NCI-H460 human lung large cell carcinoma cells in vitro. In this study, we synthesized structural analogues of 1 to explore its skeletal structure–activity relationships and found that the C18 methyl group and the configuration of the C16–C17 double bond of 1 are important for the potent antiproliferative activity. Furthermore, we prepared a series of side-chain analogues of 1 by diversification of a late-stage intermediate of our total synthesis, and found that the triene side chain of 1 could be modified to some extent without significant loss of activity, provided a Lewis basic heteroatom is placed at the terminus.

 Please wait while we load your content...
Please wait while we load your content...