An organocatalytic asymmetric double Michael cascade reaction of unsaturated ketones and unsaturated pyrazolones: highly efficient synthesis of spiropyrazolone derivatives†
Abstract
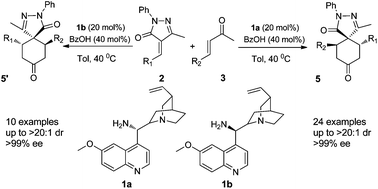
The first organocatalytic double Michael cascade reaction between unsaturated

 Please wait while we load your content...
Please wait while we load your content...