Hydroxylamine as an oxygen nucleophile: substitution of sulfonamide by a hydroxyl group in benzothiazole-2-sulfonamides†
Abstract
Benzothiazole-2-sulfonamides react with an excess of hydroxylamine in aqueous solutions to form 2-hydroxybenzothiazole, sulfur dioxide, and the corresponding amine. Mechanistic studies that employ a combination of structure–reactivity relationships, oxygen labeling experiments, and (in)direct detection of intermediates and products reveal that the reaction proceeds via oxygen attack, and that oxygen incorporated in the 2-hydroxybenzothiazole product derives from hydroxylamine. The reaction, which is performed under mild conditions, can be used as a deprotection method for cleavage of benzothiazole-2-sulfonyl-protected amino acids.
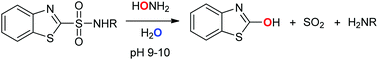

 Please wait while we load your content...
Please wait while we load your content...