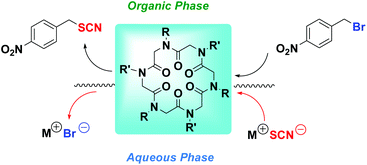
The synthesis, complexation properties and catalytic activities under phase-transfer (PT) conditions of differently substituted cyclohexapeptoids are reported. Association constants, for small cationic alkali, and catalytic performances, in a model nucleophilic substitution, are comparable to those of representative crown ethers. Noteworthy, the N-[2-(2-methoxyethoxy)ethyl] side chain derivative presents a catalytic efficiency comparable to that of crypt-222, and higher than some commonly used quaternary ammonium salts and crown ethers. Moreover its association constant for Na+ complexation proved to be higher when compared with dicyclohexyl-18-crown-6. The synthesized cyclohexapeptoids represent the first example of these peptidomimetics in PT catalysis, anticipating interesting applications in biphasic PT methodology.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...