Controlled functionalization of graphene oxide with sodium azide†
Abstract
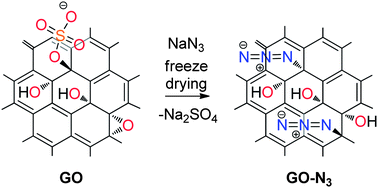
We present the first example of azide functionalization on the surface of graphene oxide (GO), which preserves thermally unstable groups in GO through the mild reaction with sodium azide in solids. Experimental evidence, by 15N solid-state NMR and other spectroscopic methods, indicates the substitution of organosulfate with azide anions as the reaction mechanism.

 Please wait while we load your content...
Please wait while we load your content...